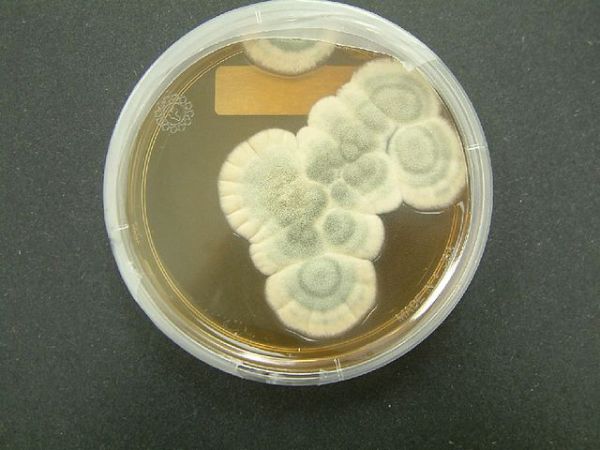
In 1928, a Scottish biologist named Alexander Fleming got very, very lucky. So lucky, in fact, that he made a discovery that would revolutionize medicine by identifying arguably the most important drug ever discovered: Penicillin. It was a discovery that, like many of our most important medicines, was possible because of natural biodiversity on the planet.
Fleming was studying the bacteria that infect human wounds when he found something that would make any microbiologist cringe: contamination in one of his bacterial cultures. Where only bacteria should have been present, he saw the tell-tale fuzz of fungus. Before he discarded the sample, he noticed that the area around this fungus was clear of bacteria, as though something about the contaminant was killing them off. Curious, Fleming investigated. Further studies showed that the fungus, eventually identified as Penicillium notatum, was producing a chemical that killed the bacteria, which Fleming named penicillin.
At the time, minor bacterial infections – like a shallow cut that festered – could be a death sentence. Fleming’s discovery, followed by the development of technology that allowed for the mass production of penicillin, changed that. Doctors now had a cheap, readily available drug that could kill off bacteria and leave their patients as good as new. Penicillin saved the lives of thousands of soldiers during World War II, and many more civilians in the years that followed.
Today, most of us take antibiotics for granted — almost everyone has been treated with them at least once in their lives. And though we rarely realize it, medicines derived from natural biodiversity have saved us from infections that could have easily killed us just fifty years ago.
We receive these antibiotics in plastic bottles of tiny capsules or in bags of clear liquid attached to intravenous lines — clinical packaging that makes them wholly unrecognizable as the creations of the natural world. And we forget that Fleming’s great discovery came from a lowly mold; that Selman Waksman, the father of antibiotics, extracted his great discoveries literally from the dirt beneath his feet. We forget that our first lines of defense, the most basic safeguards of our health, were not invented by us but, rather, by nature.
Nature is a rich source of prospective medicines for us because the existing richness of life is the product of natural selection, the process by which life evolves. Long before humans appeared on the planet, fungi began fighting a chemical war with bacteria. In what served, effectively, as a giant clinical trial, thousands of species evolved millions of different molecules with which to fight off one another. The arms race was never-ending; the price of failure was extinction. The species that are alive on our planet today represent the evolutionary winners, who house the chemical toolkit that ensures their survival in a dangerous world.
Nature’s trove of medicinal treasures includes more than antibiotics. The willow tree gave us aspirin, the pit viper’s venom can help prevent heart attacks and the periwinkle flower can treat certain types of cancer.
It’s no wonder, then, that when scientists hunt for new medicines, they often begin by screening natural compounds. Today, as concerns mount over antibiotic resistance, with methicillin-resistant Staphylococcus aureus killing 18,000 people in the United States each year, and multi-drug-resistant tuberculosis infecting almost half a million people annually, the need for new antibiotics is urgent. Research expeditions into the heart of the rainforest or to the bottom of the ocean bring back samples that are screened for antimicrobial activity. Newly discovered compounds are also tested for anti-cancer activity as we continue to seek elusive cures for that deadly set of diseases.
Successful as these approaches have been in the past, they will continue to work only so long as we have plenty of raw material to work with. At the moment, scientists estimate that we have described 15% or less of all the species currently alive on the planet. A huge potential reservoir of new medicines is contained in Earth’s biodiversity – hidden in plain view among omnipresent microbes and tucked into remote corners of the planet where humans never venture.
But this biodiversity is under threat. Humans are making Earth uninhabitable for thousands of species through the direct destruction of natural habitats, like cutting down the rainforest and polluting wetlands and through indirect changes to the function of the Earth system, like the release of greenhouse gases that alter climate. We are driving a mass extinction the likes of which hasn’t been seen since the days of the dinosaurs.
A few of these species are the charismatic polar bear or noble lion. But most are species we will never know existed, never name and never investigate. It is those species that could hold the key to the next medical breakthrough. So long as we continue to decimate Earth’s biodiversity, we will be locked in a losing race against time to catalogue these species and salvage any of their medicinal value before they are lost forever.
Holly Moeller is currently a Postdoctoral Fellow at Woods Hole Oceanographic Institution. She also writes the “Seeing Green” environmental column for The Stanford Daily.
The above post originally appeared in The Stanford Daily and is copyrighted by Holly Moeller. Contact Holly Moeller at hollyvm ‘at’ alumni.stanford.edu.
MAHB-UTS Blogs are a joint venture between the University of Technology Sydney and the Millennium Alliance for Humanity and the Biosphere. Questions should be directed to joan@mahbonline.org
The views and opinions expressed through the MAHB Website are those of the contributing authors and do not necessarily reflect an official position of the MAHB. The MAHB aims to share a range of perspectives and welcomes the discussions that they prompt.