Author(s): Gioietta Juo
Since the beginning of 2020 every aspect of our world has changed in an unrecognizable way. Over 200 countries have been affected with the COVID-19 virus. It is in every continent with the exception of the pristine Antarctica. It is now 8 months, what have we achieved? What progress has been made?. It is now time to take stock of the present situation. Are we slowly getting out of the pandemic?? What can we expect the future to look like??
DATA ON CORONAVIRUS WORLDWIDE
So far, as of September 19, 2020, the. pandemic has caused 30,906,084 cases of coronavirus disease with 959,630 deaths [1].
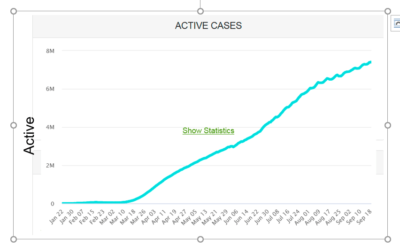
Figure 1 Active Cases in 2020 in millions
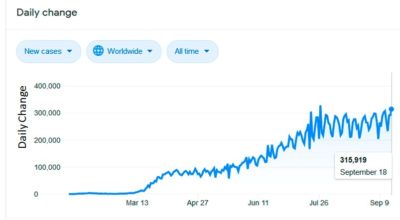
Figure 2 Daily New Cases in 2020 in thousands
It is interesting to note that only 6% of death come from the death of otherwise healthy people. The rest come from those with pre-existing conditions like diabetes, heart and organ weakness and mostly seniors in close contact in places like nursing homes.
Yes, Every aspect of our lives have changed drastically. In order to slow the spread of this highly contagious virus, countries have resorted to a drastic lockdown of ordinary social life as we know it. Schools, shops and many work places have closed. Family is confined to the home. Those who are lucky can work online at home. But others have lost their jobs and depend on government subsistence. But going out means wearing a mask for protection has been mandated.
Even though some countries have slowed down the spread of this virus with social distancing, keeping away from others by more than 6 ft, contact tracing and etc, there are potential down sides. For a start life is lonely not being able to see one’s larger family and friends. Then for those whose marriage is not rock solid, there are emotional risks like child abuse, spouse abuse, drug addictions, alcoholism, and various mental problems even suicides. It has been said that these ills as it happens are worse than the virus itself!! It is imperative that the degree to which these risks have been realized and studied so that health professionals can then develop strategies by which they can be treated. Financial problems can arise, this is where governments have come in to help small businesses and personal problems with stimulus. packages.
There is only a limited time we can lead this dreary life. It is not a permanent solution. Humans are social creatures, we need to go out, meet others, go to schools, have sports, worship in churches and so on……..Most important, schools, the economy cannot be shut down for long.
Now that the season has changed, with the sun beckoning outside, people have the urge to go outside for some fresh air, To the beaches for those living near the coast, to the national parks etc . People cannot be shut indoors forever and it is time to relax the rules.
Most importantly, schools have to open as our children need to go back to their friends and to continue their education. But how?
Social distancing is still necessary. The opening of schools has necessitated a certain closeness in living among the young students, leading to many with low grade fever. In the absence of vaccines what are the solutions? Online teaching is definitely here to stay even though it puts more stress on the families. Then there is nerd immunity [2] where a majority of people who have been exposed and acquired immunity for the virus can impart the immunity to the whole community. That is once a threshold of immune people exist hereby reducing the likelihood of infection for individuals who lack immunity. Immure individuals are unlikely to contribute to disease transmission, They disrupt the chain of infection, which stops or slows the spread of the disease. The greater the proportion of immune individuals in a community, the smaller the probability that that non-immune individuals will come into contact with an immune individual. However, the basic concepts of social distancing, cleanliness of personal hygiene still apply[2].
Definitely, small business – restaurants, hairdressers, stores and workplaces which are the backbone of a country’s economy have to open so long as the basic rules are observed. Innovative ideas such as using the sidewalk for business have sprung up.
PATH TAKEN BY CROATIA TO COMBAT COVID-19
What is happening is Croatia may be an example of what might come [3].
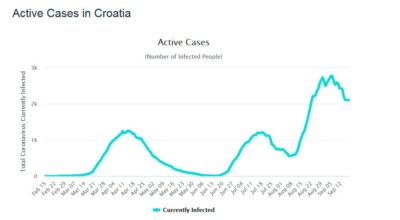
Figure 3 Active Cases in Croatia in 2020
First comes the main peak of active cases. After a mandated social distancing and general lockdown, the number of cases drops drastically. Now is the time to reopen the society and economy? However, after some social mixing, the number of cases rises again. Another lockdown has to happen. Again the number of cases drops. Another attempt of reopening happens followed by an expected rise again of the number of cases. Each time the number of people catching the disease is expected to be lower, Several attempts of reopening will happen until the disease is finally eradicated and society gets back to normal.
Having the confidence that the virus has been licked, the government decided to open up the country completely without restrictions.
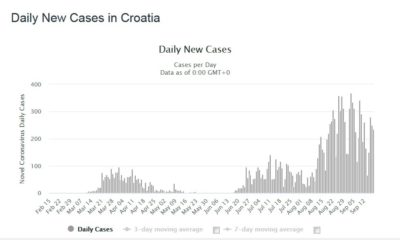
This has led to a disastrous sharp rise in new cases in a third wave. See figures 3 and 4. In fact what we are seeing is the second wave followed by the third wave. Not many countries in the world have seen such pronounced multiple waves. Spain is now seeing the second wave. There are signs that the USA is on its second wave.
Figure 4 Daily New Cases in Croatia
TREATMENTS FOR COVID-19 [4]
Although there is no product approved by the US FDA there are many drugs being tested and used. Remdesivir may be prescribed for emergency use. Otherwise the following are actively being tested:
- Antivirial drugs
In addition to Remdesivir, there are favipiravir and merimepodib.
– Dexamethasone
It is a corticosteroid anti – inflammatory drug studied to treat or prevent organ dysfunction and lung injury from inflammation. With people on ventilators or supplemental oxygen. This can reduce death by 30 %.
- Anti- inflammatory therapy
This is in general useful for more severe cases
- Immune – based therapy[4]
This is a developing therapy which has been found to be highly effective. Recently the US Food and Drug Administration has issued emergency use authorization to treat hospitalized COVID-19 patients with convalescent plasma from people who have recovered from the virus. Convalescent plasma is the liquid portion of the blood that contains the antibodies an individual develops in response to an infection and can be given to patients currently fighting that virus. This treatment has long been a part of the infectious disease arsenal. It has already been in use for COVID-19 for a number of months: The Mayo Clinic has run an “expanded access program for convalescent plasma since March, and more than 70,000 people have received the treatment. It is found that there is a 35% improvement in mortality rate for COVID-19 patients given the plasma.
- Hydroxychloroquine and chloroquine.
This is a long standing anti -malarial drug which has been used for nearly a century. However, there is a fraction of the medical community which maintain this is not an effective solution. In fact there are many people who have used it for long periods just for the prevention of malaria. For them no ill effects have been observed. So this has led to an almost political dialogue. Some say it may cause heart problems but otherwise it has been widely used across all continents with no serious effects.
- Ventilators and oxygen supplements may be used for breathing
VACCINES FOR COVID-19 [5]
It is only natural that we resort to a universal vaccine to solve the pandemic problem. But the scale of the problem given the population size of each country is gigantic. More than 150 companies are desperately competing working drastically to produce a vaccine by the end of 2020. Following are the prominent candidates but which will succeed?
The basic idea of all those vaccines is to instruct one’s immune system to mount a defense, which is sometimes stronger than what would be provided through natural infection and hopefully comes with fewer health consequences.
To do so, some vaccines use the whole coronavirus, but in a killed or weakened state. Others use only part of the virus – whether protein or a fragment. Some transfer the protein into a different virus.
Finally some use pieces of the virus’s genetic material so as to temporarily make the right proteins to stimulate the immune system.
Even when a vaccine has been chemically produced, it faces still a tortuous path to the final usable product. Vaccines have to go through a multi – stage clinical trial process. First phase starts by checking for their safety and whether they trigger an immune response to a small group of healthy individuals. Second phase finds a wider group of those who are likely to catch the virus and to gauge how effective it is. The third phase expands the group to thousands of people to make sure it is safe and effective, given that the immune response varies by age, ethnicity or underlying health conditions.
It then goes to various regulatory agencies for approval. This may take years.
Following are some of the prominent companies. There is much in common between the various companies. Most use the SARS-CoV2 protein to trigger the immune response
Name: mRNA-1273
DNA is the gene and ~RNA gives instructions for certain proteins. A mRNA vaccine is the instruction for the SARS-CoV2 protein. Once inside the cell, the protein is made and that triggers the immune response
Who: A Massachusetts-based biotech company, in collaboration with the US National Institutes of Health.
This vaccine candidate relies on injecting snippets of a virus’s genetic material, in this case mRNA, into human cells. They create viral proteins that mimic the coronavirus, training the immune system to recognize its presence.
STATUS: The third phase has started in a deal with the Swiss company Lonza. It is hoped to manufacture up to one billion doses a year.
== Pfizer
Name: BNT162b2
WHO: One of the world’s largest pharmaceutical companies, based in New York in collaboration with German biotech BioNTech.
WHAT: Also an mRNA vaccine based on cancer vaccine.
STATUS: Currently combining phase 2 and 3 on a diverse population in 30,000 people from 39 US states and from Brazil, Argentina, and Germany. Hope to supply 1.3 billion doses by end of 2021.
Name: ChAdOx1 nCoV-19
Who: The U.K. university in collaboration with AstraZeneca.
What: Oxford’s candidate is what’s known as a viral vector vaccine, essentially a “Trojan horse” presented to the immune system. Oxford’s research team has transferred the SARS-CoV-2 spike protein—which helps the coronavirus invade cells—into a weakened version of an adenovirus, which typically causes the common cold. When this adenovirus is injected into humans, the hope is that the spike protein will trigger an immune response. AstraZeneca and Oxford plan to produce a billion doses of vaccine that they’ve agreed to sell at cost.
Status: Preliminary results from this candidate’s first two clinical trial phases revealed that the vaccine had triggered a strong immune response—including increased antibodies and responses from T-cells—with only minor side effects such as fatigue and headache. It has now moved into phase three of clinical trials, aiming to recruit up to 50,000 volunteers in Brazil, the UK, USA and South Africa.
Recently it has been found that one volunteer in the test phase of AstreZeneca has contracted inflammation of the spine. It is not known whether this is related to the vaccine or an independent coincidence. So the whole test phase has been put on hold until further investigation.
==. Sinovac
Name: CoronaVac
Who: A Chinese biopharmaceutical company, in collaboration with Brazilian research center Butantan.
What: CoronaVac is an inactivated vaccine, meaning it uses a non-infectious version of the coronavirus. While inactivated pathogens can no longer produce disease, they can still provoke an immune response, such as with the annual influenza vaccine.
Status: On July 3, Brazil’s regulatory agency granted this vaccine candidate approval to move ahead to phase three, as it continues to monitor the results of the phase two clinical trials. The first phases have so far shown that the vaccine does produce an immune response with no severe adverse effects. Preliminary results of this candidate’s earlier testing in macaque monkeys, published in Science, revealed that the vaccine produced antibodies that neutralized 10 strains of SARS-CoV-2. Phase three will recruit nearly 9,000 healthcare professionals in Brazil.
== Sinopharm
Name: None
Who: China’s state-run pharmaceutical company, in collaboration with the Wuhan Institute of Biological Products. Wuhan Institute is where the virus initially started. There has been much resentment outside China, especially in the US, that China initially limited the movement of people from Wuhan but failed to let travelers go outside internationally. In this way the virus took hold in Europe and then in USA. The spread of the virus all over the world has led to countless cases and deaths. Not to mention the economic and social disruption it has caused the whole world, China should be made accountable for the gigantic disruption and suffering it has caused to the whole planet!
What: Sinopharm is also using an inactivated SARS-CoV-2 vaccine that it hopes will reach the public by the end of 2020. Sinopharm has reported that early trials of its vaccine candidate triggered a strong neutralizing antibody response in participants, with no serious adverse effects.
Status: In mid-July, Sinopharm launched its phase three trial among 15,000 volunteers—aged 18 to 60, with no serious underlying conditions—in the United Arab Emirates. The company selected the UAE, as it has a diverse population with approximately 200 different nationalities, making it an ideal testing ground.
==. Murdoch Children’s Research Institute
Name: Bacillus Calmette-Guerin BRACE trial
Who: The largest child health research institute in Australia, in collaboration with the University of Melbourne.
What: For nearly a hundred years, the Bacillus Calmette-Guerin (BCG) vaccine has been used to prevent tuberculosis by exposing patients to a small dose of live bacteria. Evidence has emerged over the years that this vaccine may boost the immune system and help the body fight off other diseases as well. Researchers are investigating whether these benefits may also extend to SARS-CoV-2,
Status: This trial has reached phase three in Australia. It has begun a series of randomized controlled trials that will test whether BCG might work on the coronavirus as well. They aim to recruit 10,000 healthcare workers in the study.
Name: Ad5-nCoV
Who: A Chinese biopharmaceutical company.
What: CanSino has also developed a viral vector vaccine, using a weakened version of the adenovirus as a vehicle for introducing the SARS-CoV-2 spike protein to the body. Preliminary results from phase two trials have shown that the vaccine produces “significant immune responses in the majority of recipients after a single immunization.” There were no serious adverse reactions documented.
Status: Though the company is still technically in phase two of its trial, on June 25, CanSino became the first company to receive limited approval to use its vaccine in people. The Chinese government has approved the vaccine for military use only, for a period of one year.
==. The Gamaleya National Center of Epidemiology and Microbiology
Name: Sputnik V
Who: This is the only Russian vaccine research institution which is in collaboration with the state-run Russian Direct Investment Fund.
What: Gamaleya has developed a viral vector vaccine that also uses a weakened version of the common cold-causing adenovirus to introduce the SARS-CoV-2 spike protein to the body. This vaccine uses two strains of adenovirus, and it requires a second injection after 21 days to boost the immune response. Russia has not published any data from its clinical trials, but officials with the institute state that they have completed phases one and two. The researchers also claim the vaccine produced strong antibody and cellular immune responses.
Status: Despite the lack of published evidence, Russia has cleared the Sputnik V vaccine for widespread use and claimed it as the first registered COVID-19 vaccine on the market. Russia reports that it will start phase three clinical trials on August 12; the World Health Organization, however, lists the Sputnik V vaccine as being in phase one of clinical trials.
CONCLUSION
Even when a vaccine is approved, there is the problem of manufacturing, distribution, scaling up of the production and deciding who should get it first. Many vaccines go through the 4th phase of regular study. This can take long time. Then what about the cost? The US government has pledged $10 billion with Pfizer to develop 300 million doses by beginning of 2021, And World Health Organization, WHO, is aiming to deliver 2 billion doses by the end of 2021. It is truly a worldwide effort in the race to produce vaccines to fight and eradicate the pandemic. The companies are located in Australia, Russia, Germany, Brazil, Switzerland, UK, USA and of course China. We hope that the ingenuity of the world’s brilliant scientists and technicians as well as the experience and organized know how of our governments and social systems will lead us through this pandemic by the end of 2020.
Gioietta Kuo, MA at Cambridge, PhD in nuclear physics, Atlas Fellow at St Hilda’s College, Oxford and Princeton University plasma physics lab, is a research physicist. Over 70 professional articles and over 100 articles in environmental problems – in World Future Society-wfs.org, amcips.org, MAHB Stanford and other worldwide think tanks. Also in Chinese in ‘ People’s Daily’ and ‘World Environment’ – Magazine of the Chinese Ministry of Environmental Protection, and others in China. She can be reached at <kuopet@comcast.net.>
REFERENCES
[1] Coronavirus Update (Live): 23,272,847 Cases and 805,907 …
https://www.worldometers.info/coronavirus/
[2] Herd immunity and COVID-19 (coronavirus): What you need to …
https://www.mayoclinic.org/herd-immunity-and-coronavirus/art-20486808
[3] Croatia Coronavirus: 7,900 Cases and 170 Deaths …
https://www.worldometers.info/coronavirus/country/croatia/
[4] FDA Authorizes Convalescent Plasma As Emergency …
https://www.capradio.org/news/npr/story?storyid=905277083 1 day ago …
https://www.capradio.org/news/npr/story?storyid=905277083 1 day ago …
[5] https://www.mayoclinic.org/diseases-conditions/coronavirus/expert-answers/coronavirus-drugs
COVID-19 (coronavirus) drugs: Are there any that work …
[6] CORONAVIRUS UPDATE: Here’s what you should know about the vaccines in development
National Geographic 2020
https://www.nationalgeographic.com/science/health-and-human-body/human-diseases/coronavirus-vaccine-tracker-how-they-work-latest-developments-cvd/
The views and opinions expressed through the MAHB Website are those of the contributing authors and do not necessarily reflect an official position of the MAHB. The MAHB aims to share a range of perspectives and welcomes the discussions that they prompt.